Embark on a journey through the fascinating realm of stoichiometry, the cornerstone of quantitative chemistry. This chapter 11 stoichiometry study guide unravels the intricate relationships between reactants and products, empowering you to predict and analyze chemical reactions with precision.
Delve into the fundamental concepts of stoichiometry, including balanced equations, the mole concept, and Avogadro’s number. Master the art of mole-to-mole, mass-to-mass, and gas stoichiometry calculations, unlocking the ability to determine the exact quantities of reactants and products involved in chemical transformations.
Introduction to Stoichiometry

Stoichiometry is the branch of chemistry that involves the study of the quantitative relationships between reactants and products in chemical reactions. It is essential for understanding the composition of compounds, predicting the products of reactions, and determining the amounts of reactants and products involved in a given reaction.
Balanced chemical equations are essential in stoichiometry. A balanced chemical equation shows the chemical formulas of the reactants and products of a reaction, as well as the stoichiometric coefficients that indicate the relative amounts of each substance involved. The coefficients in a balanced equation ensure that the number of atoms of each element is the same on both sides of the equation, satisfying the law of conservation of mass.
The Mole Concept
The mole is the SI unit of amount of substance. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12. The mole concept is essential for stoichiometry as it allows us to relate the mass of a substance to the number of atoms or molecules it contains.
Avogadro’s Number, Chapter 11 stoichiometry study guide
Avogadro’s number is the number of elementary entities (atoms, molecules, ions, or electrons) in one mole of a substance. It is approximately 6.022 × 10 23mol -1. Avogadro’s number allows us to convert between the number of particles and the amount of substance in moles.
Mole-to-Mole Calculations

Stoichiometry is the branch of chemistry that involves the study of the quantitative relationships between reactants and products in chemical reactions. Mole-to-mole calculations are a fundamental aspect of stoichiometry and involve converting between the number of moles of reactants and products.
Converting Between Moles and Mass
To convert between moles and mass, we use the molar mass of the substance. The molar mass is the mass of one mole of a substance and is expressed in grams per mole (g/mol). The formula for converting between moles and mass is:
Mass (g) = Moles (mol) × Molar Mass (g/mol)
Moles (mol) = Mass (g) ÷ Molar Mass (g/mol)
Using Stoichiometry to Calculate the Number of Moles of Reactants and Products
Stoichiometry can be used to calculate the number of moles of reactants and products in a chemical reaction. To do this, we use the balanced chemical equation for the reaction. The coefficients in the balanced chemical equation represent the number of moles of each reactant and product involved in the reaction.
Examples of Mole-to-Mole Conversions in Real-Life Scenarios
- Calculating the amount of baking soda needed to neutralize a certain amount of acid:Baking soda (sodium bicarbonate) is commonly used to neutralize acids. To calculate the amount of baking soda needed to neutralize a certain amount of acid, we can use the balanced chemical equation for the reaction:
NaHCO3+ HCl → NaCl + CO 2+ H 2O
The coefficients in the balanced chemical equation tell us that 1 mole of baking soda reacts with 1 mole of hydrochloric acid. Therefore, to neutralize 1 mole of hydrochloric acid, we need 1 mole of baking soda.
- Calculating the amount of oxygen required for combustion:Combustion is a chemical reaction that involves the burning of a fuel in the presence of oxygen. To calculate the amount of oxygen required for combustion, we can use the balanced chemical equation for the reaction. For example, the balanced chemical equation for the combustion of methane is:
CH4+ 2O 2→ CO 2+ 2H 2O
The coefficients in the balanced chemical equation tell us that 1 mole of methane reacts with 2 moles of oxygen. Therefore, to combust 1 mole of methane, we need 2 moles of oxygen.
Mass-to-Mass Calculations: Chapter 11 Stoichiometry Study Guide
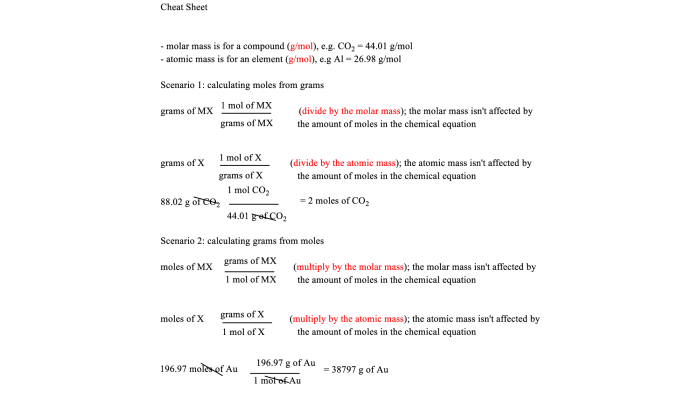
Mass-to-mass calculations in stoichiometry involve converting between the masses of reactants and products in a chemical reaction. This is useful for determining the amount of reactants or products needed or produced in a reaction.
To convert between mass and mass, we use the molar mass of the substance. The molar mass is the mass of one mole of the substance, expressed in grams per mole (g/mol). The molar mass can be found on the periodic table or in a reference book.
Example
For example, let’s say we want to convert 10.0 g of sodium chloride (NaCl) to grams of chlorine (Cl). The molar mass of NaCl is 58.44 g/mol, and the molar mass of Cl is 35.45 g/mol. We can use the following equation to convert the mass of NaCl to the mass of Cl:
“`mass of Cl = mass of NaCl × (molar mass of Cl / molar mass of NaCl)“““mass of Cl = 10.0 g × (35.45 g/mol / 58.44 g/mol)“““mass of Cl = 6.06 g“`
Therefore, 10.0 g of NaCl contains 6.06 g of Cl.
Limiting Reactant
In a chemical reaction, the limiting reactant is the reactant that is completely consumed, limiting the amount of product that can be formed. To determine the limiting reactant, we compare the mole ratio of the reactants to the stoichiometric ratio of the balanced chemical equation.
For example, consider the following reaction:
“`
H2 + O2 → 2H2O
“`
If we have 2 moles of H2 and 1 mole of O2, we can calculate the mole ratio as follows:
“`mole ratio of H2 = 2 moles H2 / 2 moles H2 = 1“““mole ratio of O2 = 1 mole O2 / 1 mole O2 = 1“`
Comparing the mole ratio of the reactants to the stoichiometric ratio of the balanced chemical equation, we see that the mole ratio of O2 is less than the stoichiometric ratio. Therefore, O2 is the limiting reactant in this reaction.
Gas Stoichiometry

Gas stoichiometry examines the quantitative relationships between the volumes, pressures, temperatures, and amounts of reactants and products in gaseous reactions. It plays a crucial role in various fields, including chemistry and environmental science.
Relationship between Gas Volume, Pressure, Temperature, and Moles
The behavior of gases is governed by the Ideal Gas Law, which establishes the relationship between these four parameters:
PV = nRT
- P represents the pressure of the gas (in pascals, Pa)
- V represents the volume of the gas (in cubic meters, m 3)
- n represents the number of moles of the gas (in moles, mol)
- R is the ideal gas constant (8.314 J/mol·K)
- T represents the temperature of the gas (in kelvins, K)
Gas Stoichiometry Calculations
Gas stoichiometry calculations involve using the Ideal Gas Law to determine the unknown quantity (volume, pressure, temperature, or moles) based on the known values of the other parameters. Common types of calculations include:
- Volume-to-volume calculations:Determine the volume of a gas at a different set of conditions using the ratio of moles and the Ideal Gas Law.
- Pressure-to-volume calculations:Determine the pressure of a gas at a different set of conditions using the ratio of moles and the Ideal Gas Law.
- Temperature-to-volume calculations:Determine the temperature of a gas at a different set of conditions using the ratio of moles and the Ideal Gas Law.
- Mole-to-volume calculations:Determine the number of moles of a gas present in a given volume at specific conditions using the Ideal Gas Law.
Applications of Gas Stoichiometry
Gas stoichiometry finds applications in various fields, including:
- Chemistry:Determining the stoichiometry of gaseous reactions, predicting the products and their quantities, and analyzing the behavior of gases in various processes.
- Environmental science:Monitoring and controlling gaseous emissions, assessing air quality, and studying the impact of gases on the environment.
- Industrial processes:Optimizing gas-related processes in industries, such as combustion, gas separation, and pollution control.
Solution Stoichiometry

Solution stoichiometry involves the quantitative study of the relationships between reactants and products in solution. It is based on the concept of molarity, which is a measure of the concentration of a solution.
Molarity is defined as the number of moles of solute per liter of solution. It is expressed in units of moles per liter (mol/L) or molar (M). The molarity of a solution can be calculated using the following formula:
Molarity = Moles of solute / Volume of solution (in liters)
Preparing Solutions of Specific Concentrations
To prepare a solution of a specific concentration, it is necessary to dissolve a known mass of the solute in a known volume of solvent. The mass of the solute can be calculated using the following formula:
Mass of solute = Molarity × Volume of solution (in liters) × Molecular weight of solute
Once the mass of the solute has been calculated, it can be dissolved in the solvent to create the desired solution.
Applications of Solution Stoichiometry
Solution stoichiometry has a wide range of applications in analytical chemistry, including:
- Determining the concentration of unknown solutions through titration experiments.
- Preparing solutions of known concentrations for use in various chemical reactions.
- Calculating the amount of reactants and products involved in chemical reactions.
Advanced Stoichiometry Applications
Stoichiometry is a fundamental concept in chemistry that extends beyond mole-to-mole and mass-to-mass calculations. It finds extensive applications in chemical kinetics, equilibrium, industrial processes, and environmental chemistry, aiding in the comprehension of complex chemical systems.
In chemical kinetics, stoichiometry helps determine the rate of reactions and predict the products formed. By understanding the stoichiometric ratios of reactants and products, scientists can use rate laws to quantify the reaction rates and predict the time it takes for a reaction to reach completion.
Equilibrium
Stoichiometry also plays a crucial role in chemical equilibrium. By analyzing the stoichiometric coefficients of a balanced chemical equation, scientists can determine the equilibrium constant and predict the relative amounts of reactants and products at equilibrium. This knowledge is essential for understanding and controlling chemical processes, such as those involving gas mixtures or acid-base reactions.
Industrial Processes
In industrial chemistry, stoichiometry is indispensable for optimizing chemical processes and maximizing product yield. For example, in the Haber process for ammonia production, stoichiometry ensures the correct ratio of nitrogen and hydrogen gases to achieve high conversion rates and minimize waste.
Similarly, in the production of plastics, stoichiometry helps determine the precise amounts of monomers and catalysts needed for polymerization reactions.
Environmental Chemistry
Stoichiometry is vital in environmental chemistry for assessing the impact of pollutants and developing strategies for pollution control. By understanding the stoichiometric relationships between pollutants and environmental components, scientists can predict the fate and transport of pollutants in the environment.
This knowledge informs decision-making regarding emission standards and remediation efforts.
Question & Answer Hub
What is the significance of stoichiometry in chemistry?
Stoichiometry provides a quantitative framework for understanding and predicting chemical reactions, allowing chemists to determine the exact amounts of reactants and products involved.
How do I perform mole-to-mole conversions?
To convert between moles and mass, use the molar mass of the substance. To convert between moles and volume for gases, use the ideal gas law.
What is the limiting reactant concept?
The limiting reactant is the reactant that is completely consumed in a reaction, limiting the amount of product that can be formed.